The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. An amu is defined as exactly (1/12 ) of the mass of a carbon-12 atom and is equal to 1.6605 ( times ) 10 −24 g.
Learning Objectives
- To determine the formula mass of an ionic or molecular compound.
- Potassium is the seventh most abundant element in the Earth's crust, accounting for about 2.5% of its mass. Element number 19 is the eighth most abundant element in the human body, accounting for between 0.20% and 0.35% of body mass. Potassium is the second lightest (least dense) metal after lithium.
- The relative atomic mass of an element is the average mass of the naturally-occurring isotopes of the element relative to the mass of an atom of 12C. For example, the atomic mass of iron is 55.845 u.
- Potassium-39 is normally about 13.5 times more plentiful than potassium-41. The natural radioactivity of potassium is due to beta radiation from the potassium-40 isotope (10 9 years half-life). The disintegration of potassium-40 is used in geological age calculations (see potassium-argon dating).
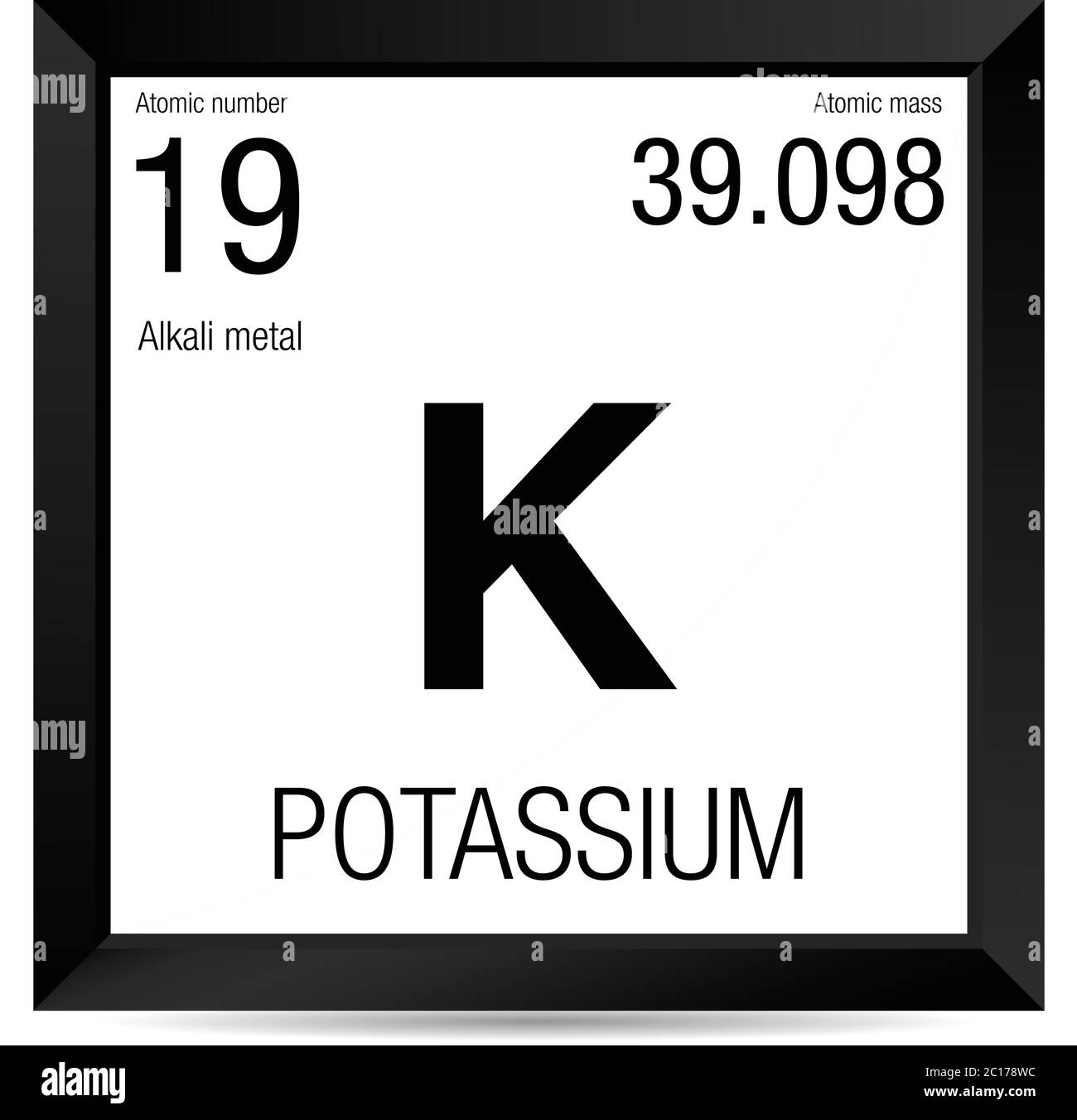
- Mass numbers of typical isotopes of Potassium are 39; 41. Atomic Mass of Potassium. Atomic mass of Potassium is 39.0983 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.
A necessary skill for future chapters is the ability to determine the mass of the formula of an ionic compound. This quantity is called the formula mass. The formula mass is obtained by adding the masses of each individual atom in the formula of the compound. Because a proper formula is electrically neutral (with no net electrons gained or lost), the ions can be considered atoms for the purpose of calculating the formula mass.
Let us start by calculating the formula mass of sodium chloride (NaCl). This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we find from the periodic table; here, we use the masses to two decimal places:
Na: 22.99 amu
Cl: +35.34 amu
Total: 58.44 amu
To two decimal places, the formula mass of NaCl is 58.44 amu.
When an ionic compound has more than one anion or cation, you must remember to use the proper multiple of the atomic mass for the element in question. For the formula mass of calcium fluoride (CaF2), we must multiply the mass of the fluorine atom by 2 to account for the two fluorine atoms in the chemical formula:
Ca: 1 x 40.08 = 40.08 amu
F: 2 x 19.00 = +38.00 amu
Total = 78.08 amu
The formula mass of CaF2 is 78.08 amu.
For ionic compounds with polyatomic ions, the sum must include the number and mass of each atom in the formula for the polyatomic ion. For example, potassium nitrate (KNO3) has one potassium atom, one nitrogen atom, and three oxygen atoms:
K: 1 x 39.10 = 39.10 amu
N: 1 x 14.00 = +14.00 amu
O: 3 x 16.00 = +48.00 amu
Total = 101.10 amu
The formula mass of KNO3 is 101.10 amu.
Potassium nitrate is a key ingredient in gunpowder and has been used clinically as a diuretic.
When a formula contains more than one polyatomic unit in the chemical formula, as in Ca(NO3)2, do not forget to multiply the atomic mass of every atom inside of the parentheses by the subscript outside of the parentheses. This is necessary because the subscript refers to the entire polyatomic ion. Thus, for Ca(NO3)2, the subscript 2 implies two complete nitrate ions, so we must sum the masses of two (1 × 2) nitrogen atoms and six (3 × 2) oxygen atoms, along with the mass of a single calcium atom:
Ca: 1 x 40.08 = 40.08 amu
N: 2 x 14.00 = +28.00 amu
O: 6 x 16.00 = +96.00 amu

Total = 164.08 amu
The key to calculating the formula mass of an ionic compound is to correctly count each atom in the formula and multiply the atomic masses of its atoms accordingly.
Example (PageIndex{1})
Use the atomic masses (rounded to two decimal places) to determine the formula mass for each ionic compound.
- FeCl3
- (NH4)3PO4
Solution
a.
Fe: 1 x 55.85 = 55.85 amu

Cl: 1 x 35.45 = +106.35 amu
________________________
Total = 162.20 amu
The formula mass of FeCl3 is 162.2 amu.
b. When we distribute the subscript 3 through the parentheses containing the formula for the ammonium ion, we see that we have 3 nitrogen atoms and 12 hydrogen atoms. Thus, we set up the sum as follows:
N: 3 x 14.00 = 42.00 amu
H: 12 x 1.00 = +12.00 amu
P: 1 x 30.97 = +30.97 amu
O: 4 x 16.00 = +64.00 amu
Total = 148.97 amu
The formula mass for (NH4)3PO4 is 149.0 amu.
Exercise (PageIndex{1})
Use the atomic masses (rounded to two decimal places) to determine the formula mass for each ionic compound.
- TiO2
- AgBr
- Au(NO3)3
- Fe3(PO4)2
Answer
a. 79.87 amu
b. 187.77 amu
c. 383.0 amu
To Your Health: Hydrates
Some ionic compounds have water (H2O) incorporated within their formula unit. These compounds, called hydrates, have a characteristic number of water units associated with each formula unit of the compound. Hydrates are solids, not liquids or solutions, despite the water they contain.
To write the chemical formula of a hydrate, write the number of water units per formula unit of compound after its chemical formula. The two chemical formulas are separated by a vertically centered dot. The hydrate of copper(II) sulfate has five water units associated with each formula unit, so it is written as CuSO4•5H2O. The name of this compound is copper(II) sulfate pentahydrate, with the penta- prefix indicating the presence of five water units per formula unit of copper(II) sulfate.
Hydrates have various uses in the health industry. Calcium sulfate hemihydrate (CaSO4•½H2O), known as plaster of Paris, is used to make casts for broken bones. Epsom salt (MgSO4•7H2O) is used as a bathing salt and a laxative. Aluminum chloride hexahydrate is an active ingredient in antiperspirants. The accompanying table lists some useful hydrates.
| Formula | Name | Uses |
|---|---|---|
| AlCl3•6H2O | aluminum chloride hexahydrate | antiperspirant |
| CaSO4•½H2O | calcium sulfate hemihydrate (plaster of Paris) | casts (for broken bones and castings) |
| CaSO4•2H2O | calcium sulfate dihydrate (gypsum) | drywall component |
| CoCl2•6H2O | cobalt(II) chloride hexahydrate | drying agent, humidity indicator |
| CuSO4•5H2O | copper(II) sulfate pentahydrate | fungicide, algicide, herbicide |
| MgSO4•7H2O | magnesium sulfate heptahydrate (Epsom salts) | laxative, bathing salt |
| Na2CO3•10H2O | sodium carbonate decahydrate (washing soda) | laundry additive/cleaner |
Key Takeaway
- Formula masses of ionic compounds can be determined from the masses of the atoms in their formulas.
Contributors and Attributions
Anonymous
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
››Convert moles Potassium to gram
Please enable Javascript to usethe unit converter.
Note you can turn off most ads here:
https://www.convertunits.com/contact/remove-some-ads.php
››More information from the unit converter
How many moles Potassium in 1 grams?The answer is 0.025576559594663.
We assume you are converting between moles Potassium and gram.
You can view more details on each measurement unit:
molecular weight of Potassium orgrams
The molecular formula for Potassium is K.
The SI base unit for amount of substance is the mole.
1 mole is equal to 1 moles Potassium, or 39.0983 grams.
Note that rounding errors may occur, so always check the results.
Use this page to learn how to convert between moles Potassium and gram.
Type in your own numbers in the form to convert the units!
››Quick conversion chart of moles Potassium to grams
1 moles Potassium to grams = 39.0983 grams
Atomic Mass Of Potassium Rounded
2 moles Potassium to grams = 78.1966 grams
3 moles Potassium to grams = 117.2949 grams
Atomic Mass Of Potassium Bromide
4 moles Potassium to grams = 156.3932 grams
5 moles Potassium to grams = 195.4915 grams
6 moles Potassium to grams = 234.5898 grams
7 moles Potassium to grams = 273.6881 grams
8 moles Potassium to grams = 312.7864 grams
Atomic Mass Of Potassium In Grams
9 moles Potassium to grams = 351.8847 grams
10 moles Potassium to grams = 390.983 grams

››Want other units?
You can do the reverse unit conversion fromgrams Potassium to moles, or enter other units to convert below:
››Common amount of substance conversions
moles Potassium to nanomol
moles Potassium to centimol
moles Potassium to picomol
moles Potassium to kilomol
moles Potassium to decimol
moles Potassium to atom
moles Potassium to millimol
moles Potassium to mole
moles Potassium to molecule
moles Potassium to micromol
Atomic Mass Of Potassium And Manganese
››Details on molecular weight calculations
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
Atomic Mass Of Potassium Carbonate
››Metric conversions and more
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!
