- A particle undergoes three successive displacements in a plane as follows: 4.0m southwest, 5.0m east, 6.0 m in a direction 60o north of east.
- The energy level of an atom's valence electrons correspond to its period or horizontal row on the periodic table. Hydrogen and helium, both in the first period, have their valence electrons in the.
The electrons of an atom are typically divided into two categories: valence and core electrons. Valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy levels. This difference greatly influences the role of the two types of electrons in a chemical reaction. Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom.
The #'octet rule'# determines the number of valence electrons in the noble gases, excluding He. Noble gases have the general electronic configuration #ns^2np^6#. Noble gases are typically nonreactive. They do, however, have some cool applications e.g. Step 4: Find Valence Electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of four electrons present in the valence shell of carbon (2s 2 2p 2). Thus, carbon has four valence electrons. Valency of Carbon (C).
The electron configuration of a oxygen atom is
[ce{O}: ,1s^22s^22p^4 label{1}]
which may be shorted
[ce{O}:, [He]2s^2 2p^4 label{2}]
where the ([He]) stands for the configuration of helium ((1s^2)). Similarly, the configuration of calcium with 20 electrons can be written
[ce{Ca}:, [Ar]4s^2 label{3}]
Valence Electrons In Sodium
where the ([Ar]) stands for the configuration of argon ((1s^22s^22p^6 3s^2 3p^6)). Electronic configurations that are the same as noble gases are very stable since they have a full octet (except helium with a full 1s orbital).
The (1s) electrons in oxygen do not participate in bonding (i.e., chemistry) and are called core electrons. The valence electrons (i.e., the (2s^22p^4) part) are valence electrons, which do participate in the making and breaking of bonds. Similarly, in calcium (Equation (ref{3})), the electrons in the argon-like closed shell are the core electrons and the the two electrons in the 4s orbital are valence electrons.
Example (PageIndex{1}): Cobalt
What are the core and valence electrons in cobalt?
Solution
Start by writing the electron configuration of cobalt with 27 electrons:
[1s^22s^22p^63s^23p^64s^23d^7 nonumber]
However, argon has the electronic structure (1s^22s^22p^23s^23p^6), so we can rewrite the configuration as
[[Ar]4s^23d^7 nonumber]
The two electrons in the (4s) orbital and the seven electrons in the (3d) are the valence electrons: all others are core electrons.
The periodicity of valance electrons can be seen in the Periodic Table. Basically, the periodicity is only applied to the main group elements, while in transition metals, rules are complex.
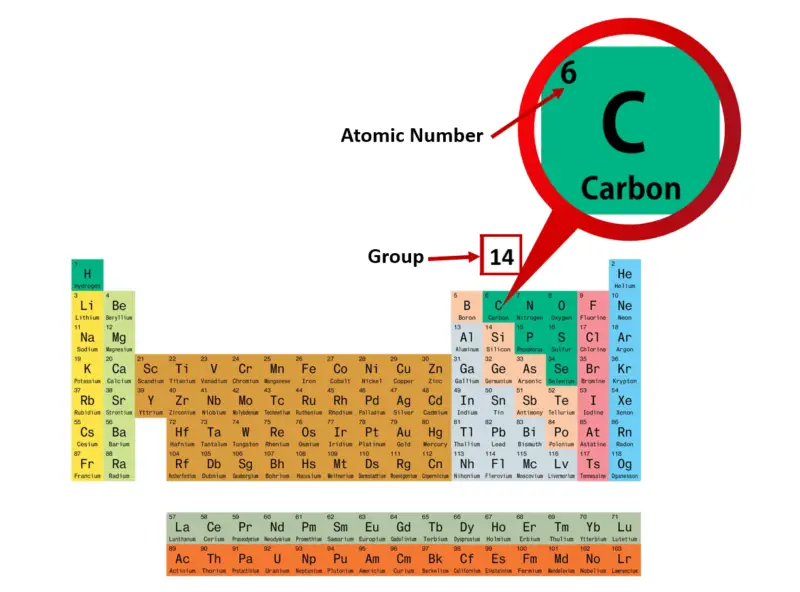
The core electrons remain the same in the increase of group numbers in the main group elements. On the other hand, the valance electrons increase by one from left to right of a main period, and remain the same down the column of a main group. This evolution gives periodical change in property of a period, and similar chemical property of a group, which is called periodical trend. The number of valence electrons in a main period is the same as its group number. The table below shows this rule clearly.
Under construction
Figure 1: 1A + 2A are metals. 3A to 8A are non-metals.
However, this periodicity cannot be applied to the transition group, which is more complicated than that of the main group. Although the outermost electrons can be easily determined, the apparent valence electrons considered in chemical reactivity are complex and fluctuated. Electrons going into d sublevel can play either a role of valence electrons or shielding electrons. So there is not always a certain number of apparent valence electrons. The number of apparent valence electrons for the first transition metal period is shown in the table below.
Under construction

Figure 2: Valence electrons for transition metals.
Relationship with Chemical Reactivity
The chemical reactivity of an atom is mainly determined by valence electrons. Atoms which have a complete shell of valence electrons tend to be chemically inert. Atoms with one or two valence electrons are highly reactive. This phenomenon can be explained by Hund's rule, which states that orbitals that are empty, half-full, or full are more stable than those that are not. For example, Ne is chemically inert because it has two valence electrons that fill its outermost shell which makes it stable compared to atoms such as Al, which has three valence electrons, but its valence electrons does not fill its outermost shell.
Although core electrons do not take part in chemical bonding, they play a role in determining the chemical reactivity of an atom. This influence is generally due to the effect it has on valence electrons. The effect can be observed from the gradual change of chemical reactivity in a group. As you go down a group, more shells are occupied by electrons, which increases the size of the atom. The more core electron shells an atom has, the larger the size of the atom, and the farther the valence electrons are from the nucleus, thus the valence electrons will experience less effective nuclear charge and will be easily lost. For example, (ce{Na}) and (ce{K}) can both react with water, but K has a more radical reaction because it has more shells of core electrons which makes the valence electron in its outermost orbital much easier to lose than the valence electron of Na.
References
- Miessler, Gary L., and Donald A. Tarr. Inorganic Chemistry. Upper Saddle River, NJ: Pearson Prentice Hall, 2010. Print.
- Brown, Ian David. The Chemical Bond in Inorganic Chemistry the Bond Valence Model. Oxford: Oxford UP, 2006. Print.
Valence electrons are those electrons in the outer shell of a given atom, the number of which determines how atoms interact with each other. An atom is said to have a closed shell when it has enough valence electrons in order to make it stable; when there are not enough, it is said to have an open shell. An atom with an open shell is constantly trying to reach stability, forming one of the foundations of many chemical reactions.
An atom is reactive or inert depending on how many valence electrons it has. The most reactive atoms are those that have one or two to lose or those that have one or two to gain in order to maintain stability. For this reason, the noble gases — all of which have a closed outer shell in nature — are chemically inert. In general, eight electrons are needed for an atom to reach stability. Two notable exceptions are hydrogen and helium, both of which need two to make a closed shell.
The affinity for atoms to achieve stability by gaining or losing valence electrons provides a foundation for two types of chemical bonds: the ionic bond and the covalent bond. Ionic bonds are formed when one atom “steals” an electron from another. Table salt (NaCl) is an example of this. Sodium (Na) has one electron to give up. Chlorine (Cl), on the other hand, needs one to be complete.

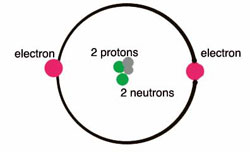
Total Number Of Valence Electrons In Helium
To reach stability, chlorine will take an electron from sodium. This allows both elements to achieve a closed shell and stability. The result of this is that the sodium atom becomes a positive ion and the chlorine atom becomes a negative ion. The opposite charges will then attract each other. When in a solution, these molecules also conduct electricity, since ions are free to move around in the solution.
I Valence Electron
Water is an example of atoms forming a covalent bond. Hydrogen has one atom to gain or lose and oxygen needs two to achieve stability. In this application, however, the oxygen does not steal the electrons from the two hydrogen atoms. Rather, the oxygen and the two hydrogen atoms share the electrons, forming a water molecule. Atoms can also use covalent bonds to share electrons with atoms of the same element, such as in a hydrogen molecule (H2).
