Avogadro’s number, which gives the number of molecules or atoms in a mole of any substance. All electrons have a constant charge of 1.602.10-19 C. If you measure the current of this system for a certain amount of time, you can determine the total charge passed through the wire, thus determine the total number of electrons that were involved. The purpose of this lab was to give us a different perspective of where Avogadro’s number comes from. We learn in a hands-on approach how it can be determined by the unique properties of oleic acid, water, and pentane. The first step in this lab was to achieve a properly diluted solution of oleic acid.
Due to the incredibly small size of the basic particles of matter there are fantastic numbers of them in the masses used in everyday life. Amedeo Avogadro is the scientist most credited with work done in this domain. | Amedeo Avogadro |
Avogadro's Number Calculator
Avogadro's number
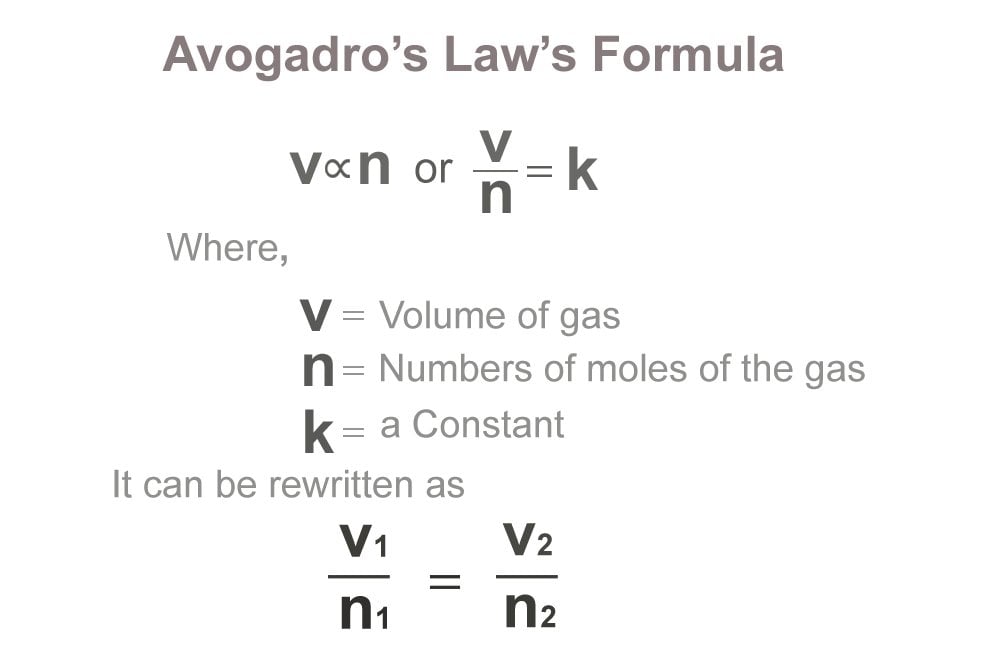
Avogadro’s law tells about the relationship between the volume of a gas and the number of molecules possessed by it. It was formulated by an Italian scientist Amedeo Avogadro in the year 1811. During a series of experiments conducted by him, he observed that an equal volume of gases contains an equal number of particles.
Avogadro defined the number of particles of a substance that have a mass equivalent to the relative mass of that substance. He found that the number of atoms in 12 g of carbon was equal to approximately 6.02 x 1023
Avogadro's Number Practice
This number was given his name - the Avogadro constant.
Avogadro's number = 6.02 x 1023
An Avogadro number of particles = 6.02 x 1023 particles
The Avogadro number or constant may be represented by the letter 'L' and when dealing with this number of particles we say that there is an 'amount' of 1 mole.


1 mole of any substance contains an Avogadro number of particles of that substance = 6.02 x 1023 particles
Examples: 1 mole of iron contains 6.02 x 1023 iron atoms 1 mole of chlorine gas contains 6.02 x 1023 chlorine molecules 1 mole of electrons contains 6.02 x 1023 electrons (also called a Faraday of charge) |
Define Avogadro's Number With Example
Note that the Avogadro constant simply describes a specific number of any particle, be they atoms, molecules, electrons or even armchairs!

